Why XADAGO?

Why XADAGO?
Levodopa has
limitations over time
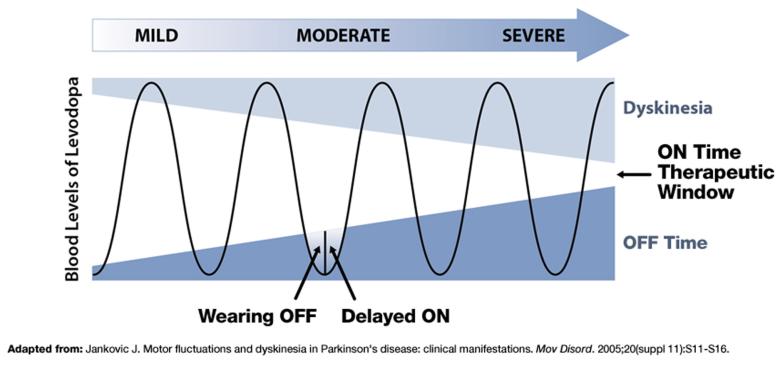
Despite its efficacy, the therapeutic window
of levodopa narrows over time1,2
Inconsistent levodopa response increases the need for adjunctive treatment options3
Mechanism of Action
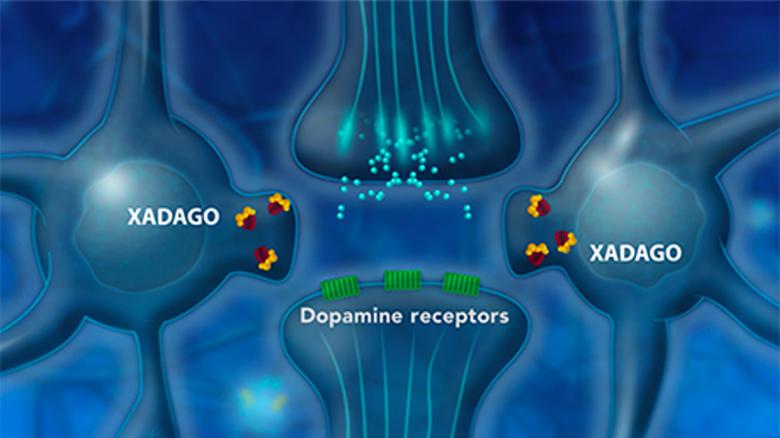
XADAGO is a highly selective inhibitor of monoamine oxidase B (MAO-B)4,5
- XADAGO has over 1000-fold greater selectivity for MAO-B over MAO-A4
- The precise mechanism by which XADAGO exerts its effects in Parkinson’s disease (PD) is unknown4
Identifying XADAGO patients
These are hypothetical patients who may be appropriate for treatment with XADAGO.
Carl, 62-year-old male, department manager at grocery store

DIAGNOSIS
Parkinson’s disease at age 57
CURRENT TREATMENT
Currently taking carbidopa/levodopa (25 mg/100 mg) 3x/day and pramipexole extended-release 2.25 mg
CHALLENGES
- Reports his carbidopa/levodopa dose is wearing off sooner
- Recently, dyskinesia and difficulty walking are impairing his work and ability to play with his grandchildren even after taking his medication
- He is unable to increase pramipexole due to lower-extremity edema at higher dose
See how XADAGO can help patients experiencing motor fluctuations
DIAGNOSIS
Parkinson’s disease at age 57
CURRENT TREATMENT
Currently taking carbidopa/levodopa (25 mg/100 mg) 3x/day and pramipexole extended-release 2.25 mg
CHALLENGES
- Reports his carbidopa/levodopa dose is wearing off sooner
- Recently, dyskinesia and difficulty walking are impairing his work and ability to play with his grandchildren even after taking his medication
- He is unable to increase pramipexole due to lower-extremity edema at higher dose
See how XADAGO can help patients experiencing motor fluctuations
Susan, 66-year-old female, nursing director at a community hospital

DIAGNOSIS
Parkinson’s disease at age 60
CURRENT TREATMENT
Carbidopa/levodopa (25 mg/100 mg) 3x/day
CHALLENGES
- She is experiencing more OFF time between doses of carbidopa/levodopa
- She reports an increase in the severity of her symptoms during ON time, including slowed movements and difficulty speaking
- She recently chose to discontinue adjunctive treatment with a dopamine agonist due to the excessive sleepiness and constipation
Learn about improving ON time—without troublesome dyskinesia
DIAGNOSIS
Parkinson’s disease at age 60
CURRENT TREATMENT
Carbidopa/levodopa (25 mg/100 mg) 3x/day
CHALLENGES
- She is experiencing more OFF time between doses of carbidopa/levodopa
- She reports an increase in the severity of her symptoms during ON time, including slowed movements and difficulty speaking
- She recently chose to discontinue adjunctive treatment with a dopamine agonist due to the excessive sleepiness and constipation
Learn about improving ON time—without troublesome dyskinesia